Which of the Following Is a Statement of Hess's Law
Which of the following is a statement of Hesss law. The enthalpy of reaction does not depend on the steps taken in the.

Solved 21 Which Of The Following Is A Statement Of Hess S Chegg Com
O The DH for a process in the forward direction is equal to the DH for the process in the reverse direction.

. Which of the following is a statement of Hesss law. If a reaction is carried out in a series of steps the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. Aif a reaction carried out in a series of steps the delta H for the reaction will equal the sum of the enthalpy changes for the individual steps.
The change of enthalpy of a reaction can be found be subtracting the enthalpy of the products from the enthalpy of the reactants. The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to the ΔH for the process in the reverse direction. A If a reaction is carried out in a series of steps the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps.
If a reaction is carried out in a series of steps the Δ H for the reaction will equal the sum of the enthalpy changes for the individual steps. A If a reaction is carried out in a series of steps the AH for the reaction will equal the sum of the enthalpy changes for the individual steps. AH involves the formation of 1 mol of substance in its standard state from its constituent elements in their standard states.
The Hesss law states that when reactants are converted to products the change in enthalpy is the same whether the. The enthalpy of reaction is determined from temperature changes in the reaction. C The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to the ΔH.
B If a reaction is carried out in a series of steps the. The enthalpy of reaction is exothermic if all the intermediate steps are exothermic. Which of the following is correct concerning hesss law.
1³³ Which of the following is a true statement of Hesss Law. If a reaction is carried out in a series of steps the Δ H for the reaction will equal the sum of the enthalpy changes for the individual steps. You will need to use fractional coefficients for some equations.
If a reaction is carried out in a series of steps the Δ H for the reaction will equal the product of the enthalpy changes for the individual steps. Bif a reaction carried out in a series of steps the delta H for the reaction will equal the product of the enthalpy changes for the individual. Which of the following is a statement of hesss lawaif a reaction carried out in a series of steps the delta H for the reaction will equal the sum of the enthalpy changes for the individual stepsbif a reaction carried out in a series of steps the delta H for the reaction will equal the product of the enthalpy changes for the individual stepscthe delta.
What is Hess law explain with example. Which of the following is a statement of Hesss law. See answer 1 Best Answer.
Which of the following is a statement of Hesss law. Which of the following is a statement of Hess Law. Hesss Law of Constant Heat Summation or just Hesss Law states that regardless of the multiple stages or steps of a reaction the total enthalpy change for the reaction is the sum of all changes.
O The DH of reaction depends on the physical states of the reactants and products. This law is a manifestation that enthalpy is a state function. The Δ H of a reaction does not depend on the physical states of the reactants and products.
For the reaction will equal the product of the enthalpy changes for the individual steps. The total enthalpy change in a chemical reaction is independent of the number and nature of intermediate steps that may be involved in that reaction. Science Chemistry QA Library Set up a Hesss law cycle and use the following information to calculate AH for aqueous nitric acid HNOtaq.
Which of the following is a statement of Hesss law. Hess law is based on the state function character of enthalpy and the first law of thermodynamics. So enthalpy of reactant and product molecules is a constant and does not.
Which of the following is a statement of Hesss law. Which of the following is a statement of hesss law. 3NO9 H01 2HNOag NOg AH 131.
The enthalpy of reaction does not depend on the steps taken in the reaction. Energy enthalpy of a system molecule is a state function. O If a reaction is carried out in a series of steps DH for the reaction will equal the sum of the enthalpy changes.
The energy of the universe is a constant. The change in enthalpy can be found be subtracting the enthalpy of the reactants from the enthalpy of the products. Hesss Law of Constant Heat Summation or just Hesss Law states that regardless of the multiple stages or steps of a reaction the total enthalpy change for the reaction is the sum of all changes.
B If a reaction is carried out in a series of steps the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps. A If a reaction is carried out in a series of steps the AH for the reaction will equal the. The Δ H for a process in the forward direction is equal to the Δ H for the process in the reverse direction.

Solved Which Of The Following Is A Statement Of Hess S Law Ifa Reaction Is Carried Out In A Series Of Steps The Ah For The Reaction Will Equal The Product Of The Enthalpy
![]()
Hess S Law Statement Illustration Application Problems Read Chemistry

Hess S Law Equation Rules Diagram What Is Hess S Law Video Lesson Transcript Study Com

Solved 24 Which Of The Following Is A Statement Of Hess S Chegg Com
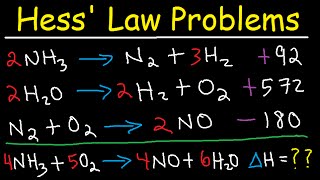
Hess Law Chemistry Problems Enthalpy Change Constant Heat Of Summation Youtube
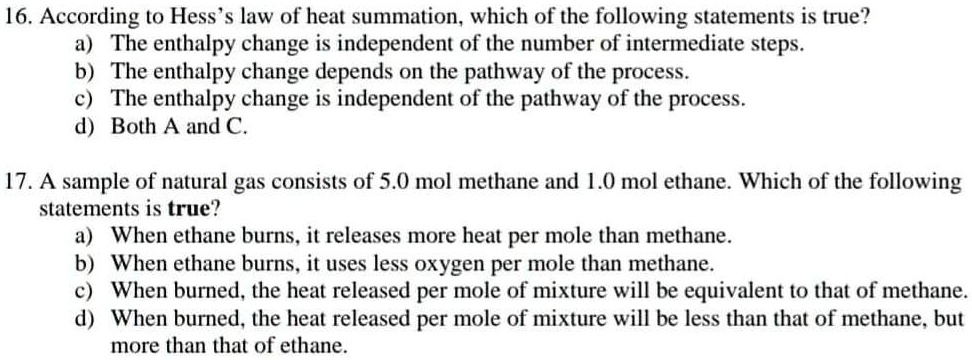
Solved 16 According To Hess S Law Of Heat Summation Which Of The Following Statements Is True A The Enthalpy Change Is Independent Of The Number Of Intermediate Steps B The Enthalpy Change Depends
Solved Heats Of Reaction Hess Law Name In This Experiment You Will Determine And Compare The Quantity Of Heat Energy Released In Three Exothermi Course Hero

Solved Question 8 Which Statement About Hess S Law Is True Chegg Com

Ib Chemistry Ellesmere College 5 2 Hess S Law

Hess Law Practice Hess S Law Practice Problems Answers Determine Ho For Each Of The Following Studocu

Hess S Law Equation Examples Chemtalk

Solved Which Of The Following Is A Statement Of Hess S Law Chegg Com

Solved 21 Which Of The Following Is A Statement Of Hess S Chegg Com

Hess S Law Statement Equation Examples What S Insight

Solved Hess S Law Virtual Lab Comparison Statement For Reactions 1 3 Hess Law Calculation And Comparison T0 The Experimental Data Difference Between The Calculated And Experimental Value Of Rxn 2 Effect Of

Which Of The Following Is A Statement Of Hess S Law Brainly Com

Solved Which Of The Following Is A Statement Of Hess S Law Chegg Com
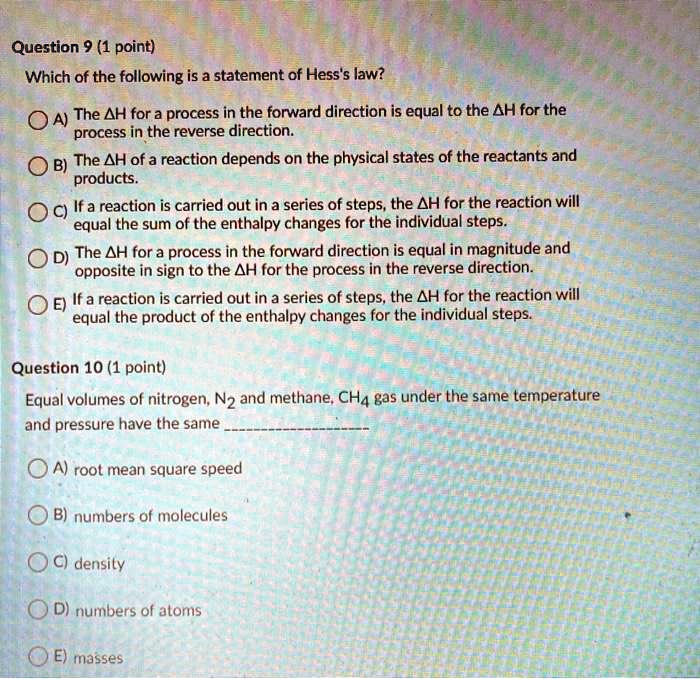
Solved Question 9 1 Point Which Of The Following Is A Statement Of Hess S Law The Ah For A Process In The Forward Direction Is Equal To The Ah For The Oa Phcafy
